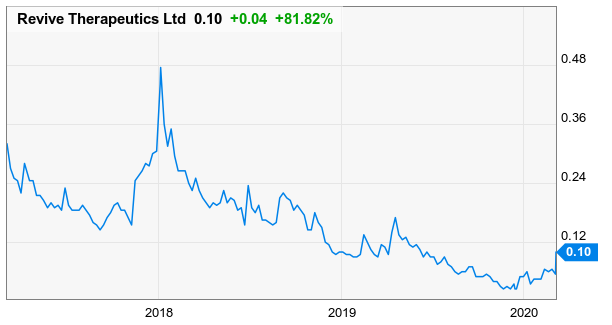
Yesterday Revive Therapeutics (CSE: RVV) increased 81.8% from 5.5c to 10c on 4.1M shares traded. Presumably, RVV is beginning to take notice, thanks to the listing of other psilocybin issuers in Canada including MindMed (NEO: MMED) and Champignon Brands (CSE: SHRM).
Prior to the recent run up, it has been a challenging couple of years for RVV shareholders which saw the stock decline from a high of 48c in early 2018 to a low of 2.5c prior to the recent run back to 10c.
In late 2019, Revive announced management changes which included Michael Frank stepping in as CEO. Shortly afterwards the company announced the acquisition of Psilocin Pharma Corp.
Psilocin Pharma focuses on intellectual property covering methods of production of psilocybin based formulations. They have developed 6 formulations to date ranging from capsules, sprays and breath strips. The precisely dosed formulations work with both natural and synthetically derived psilocybin which will be targeted for clinical research and subject to eventually FDA approval in the treatment of depression, anxiety, bi-polar disorder, bulimia & anorexia nervosa, etc.
The companies core mandate outside of the recently announced acquisition is to focus on the research, development and commercialization of novel cannabinoid-based and life sciences products. Revive’s cannabinoid pharmaceutical portfolio focuses on rare inflammatory areas such as liver disease. The company has been granted FDA orphan drug status designation for the use of CBD to treat auto-immune hepatitis (liver disease) and FDA orphan drug status designation for the use of CBD to treat ischemia and reperfusion injury from organ transplantation. They are expected to take a similar approach with Psilocin Pharma.
In a corporate update on February 16th, 2020, Revive announced they are currently in the process of preparing their Investigational New Drug (IND) application for submission to the U.S. Food and Drug Administration (FDA) for CBD in the treatment of liver disease.
In the announcement newly appointed CEO Michael Frank said, “Revive is focused on advancing its cannabinoid-based product pipeline towards human clinical studies with our lead program being CBD in the treatment of AIH (auto-immune hepatitis), a rare liver disease that represents a large market opportunity globally.”
Yesterday Revive Therapeutics closed up 81.18% on the day to $0.10.
FULL DISCLOSURE: Revive Therapeutics is a client of Canacom Group, the parent company of The Deep Dive. The author has been compensated to cover Revive Therapeutics on The Deep Dive, with The Deep Dive having full editorial control. Not a recommendation to buy or sell. Always do additional research and consult a professional before purchasing a security.









