This morning Revive Therapeutics (CSE: RVV) announced they have signed a supply agreement with Havn Life Sciences Inc. (CSE : HAVN) to source naturally-derived psychedelic compounds, such as psilocybin, for use in future investigational new drug (IND) enabling studies and clinical trials under the FDA guidelines.
Commenting on the announcement, CEO Michael Frank stated, “We are excited about our strategic partnership with Havn Life as one of our suppliers of psychoactive compounds that we intend to develop and commercialize using our established tannin-chitosan based proprietary oral-thin film delivery system, for the pharmaceutical and wellness markets.”
Havn Life Sciences is a startup biotech that describes their mission as “to unlock human potential using evidence-informed research.” They are focused on standardized, quality-controlled extraction of psychoactive compounds from plants and fungi, and the development of natural health care products from non-regulated compounds. The company has many of the noteworthy players from the cannabis world including co-CEOs Tim Moore and Susan Chappelle from Green Growth Brands and Pasha Brands. But even more noteworthy is their Chairman Vic Neufield from Aphria.

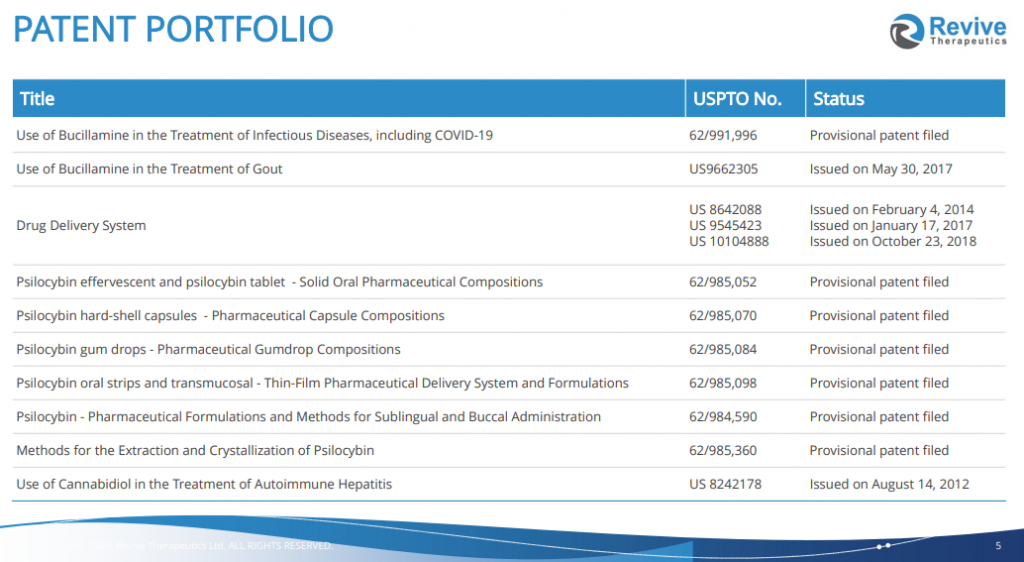
Revive has become most commonly known for their Phase 3 study to treat COVID patients with repurposed drug Bucillamine. They are now adding to their portfolio studying psilocybin, which has been in the works since last winter. Working with sponsored research partner the Reed Research Group out of the University of Wisconsin-Madison, Revive is developing its tannin-chitosan composite of orally dissolvable thin films to offer a unique delivery platform for therapeutic doses (1-20mg) of psilocybin into the oral cavity.
Revive recently entered into a Clinical Trial Agreement with the Board of Regents of the University of Wisconsin System to conduct a clinical study entitled “Phase I Study of the Safety and Feasibility of Psilocybin in Adults with Methamphetamine Use Disorder.”
Revive stock last traded down 0.5c to 25c at the time of publishing.
FULL DISCLOSURE: Revive Therapeutics is a client of Canacom Group, the parent company of The Deep Dive. The author has been compensated to cover Revive Therapeutics on The Deep Dive, with The Deep Dive having full editorial control. Not a recommendation to buy or sell. Always do additional research and consult a professional before purchasing a security.









