COVID-19 related stocks are all the rage this morning, however it appears to be for the wrong reason. Following the announcement by Pfizer this morning that it has developed a vaccine proven to be 90% effective against the disease, numerous small cap COVID-19 related stocks have cratered far more than current shareholders had previously expected.
While several of the names have since recovered marginally after hitting lows, the pain is still present with losses on the day reduced to only 30% or so after much harder falls.
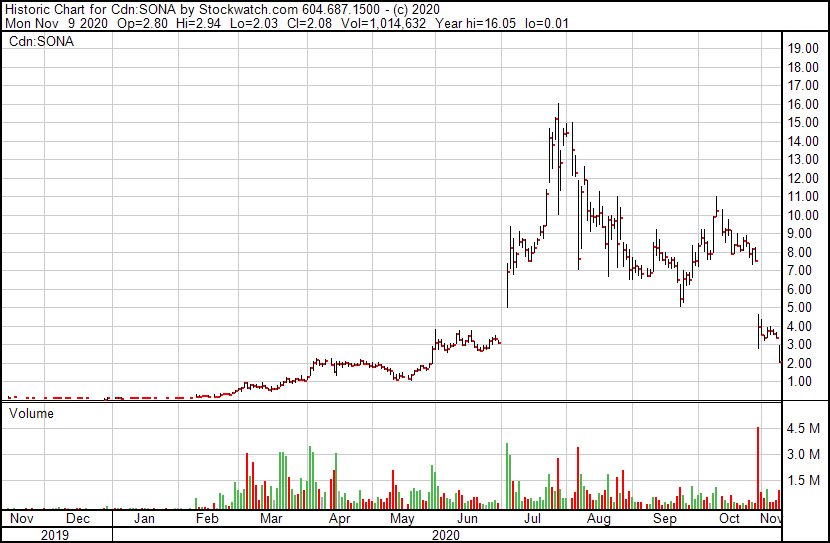
First on the list of market moving losers this morning is that of Sona Nanotech (CSE: SONA), whom is currently looking to develop a COVID-19 rapid antigen test using various gold nanoparticles. The company has been featured in multiple market movers pieces previously, however that was typically on a movement to the north. What numerous investors appear to have failed to consider in this firm, is that the development of a vaccine would be a heavily negative event for a company looking to focus on testing of the disease.
While the firm was focused on gold nanoparticles prior to the pandemic setting in, the company had little fanfare until it did a slight pivot to that of the deadly disease. It’s product is also used for diagnostic and medical applications, none of which appear to be nearly as exciting to investors as a COVID-19 rapid test. The company as a result sunk as low as $1.93 on the Pfizer news this morning, before bouncing back to $2.23 at the time of writing with ~1.30 million shares trading hands on a 34.4% decline.
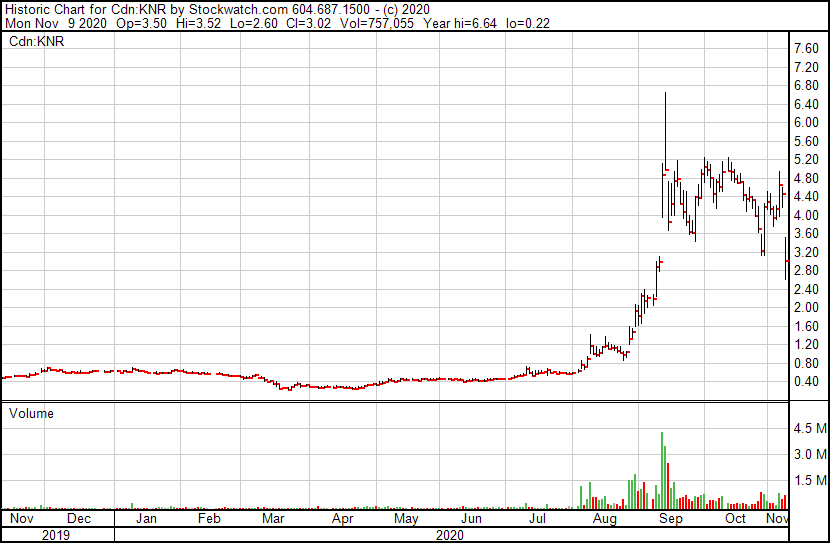
Next on the list is that of Kontrol Energy (TSXV: KNR), whom is focused on developing its BioCloud Analyzer device, which is used to detect COVID-19 particles in the air. Having recently received a CSA Standards approval for its technology, investors were adamant that the product was very near the commercialization phase, right in time for the second wave of the virus.
The company had previously projected that it could supply the market with up to 20,000 units per month, at a cost of US$12,000 per device – a figure which made investors starry eyed. This pricing also didn’t include the cost of the filter, which needs to be replaced every time it detects the virus particles. The filter itself was previously estimated to cost $300 to $400 as well.
The company just announced the lower detection limits of the virus, which sits at just 50 SARs-COV-2 virus particles. The device was designed for “places where people gather,” with sample photography most frequently locating the device in classrooms and retirement homes, while the application is said to be suitable for hospitals and mass transportation locations as well along with other locations.
However, this Pfizer vaccine development has again cooled the excitement greatly on the company. The theory here being that naturally, a vaccine will remove the need for constant detection of air for the virus.
At the time of writing, Kontrol Energy fell as low as $2.61 per share before rebounding sharply to $3.08, giving the equity a current loss of 30.9% on the day with ~900,000 shares traded.
Outside of the two headline makers, several other COVID-19 related equities have also seen plummeting share prices. For instance, Biovaxys Technology (CSE: BIOV), a firm focused on developing viral and oncology vaccine platforms for SARS-CoV-2, while also focusing on a vaccine for the disease, has fallen to $0.375 per share, down $0.215 for a 36.4% decline on 1.2 million shares.
Immunoprecise Antibodies (TSXV: IPA), whom has focused on COVID-19 research since January and is in the process of jointly developing a vaccine is off by 19.5% today following the Pfizer development. A total of ~850,000 shares have traded hands at the time of writing to take the equity down to $1.68 per share.
While it appears to be doom and gloom for these and other COVID-19 related stocks, theres a few things that should be commented on in regards to the Pfizer vaccine. First, its 90% effective – meaning that the disease likely won’t go away entirely, even if everyone were to get the vaccine. Second, the vaccine is still going through the clinical trial process, with efficacy not yet being finalized. Third, the data has yet to be peer reviewed.
The company has indicated that it expects to produce up to 50 million vaccine doses in 2020, and up to 1.3 billion doses in 2021. However, the vaccine is a two-dose type, meaning that only half of that figure is effectively how many people can initially be vaccinated. The result, is that we are far from being in the clear as of yet from the deadly disease.
Information for this briefing was found via Sedar and the companies mentioned. The author has no securities or affiliations related to this organization. Not a recommendation to buy or sell. Always do additional research and consult a professional before purchasing a security. The author holds no licenses.









