Tetra Bio Pharma (TSXV: TBP) is currently seeing upward price momentum on the discovery that one of its products appears to have been approved by Health Canada. The online Health Canada products database has listed one of the company’s products as “approved,” with the status change date being listed as January 15, 2020.

The product that has been approved for over the counter, or OTC sales is known as Terpacan Back & Muscle Pain, and is one of the first products to be approved by Health Canada that contains known cannabinoids. The product is a topical targeted towards pain relief, and has been assigned the DIN, or drug identification number, of 02495708.
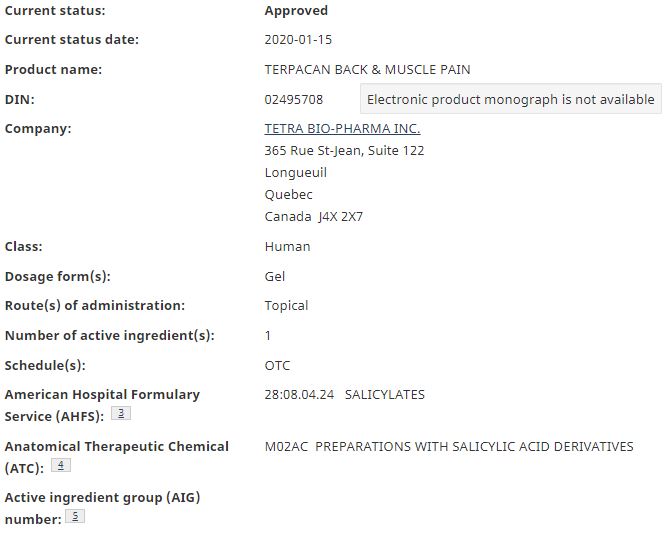
The company has yet to release an official news release on the product approval.
Tetra Bio Pharma last traded at $0.61, up 45% on the leak of the news.
Information for this briefing was found via Sedar, Health Canada and Tetra Bio-Pharma. The author has no securities or affiliations related to this organization. Not a recommendation to buy or sell. Always do additional research and consult a professional before purchasing a security. The author holds no licenses.









Market Movers: Tetra Bio-Pharma
Cannabis adjacent pharma-co Tetra Bio-Pharma (TSXV: TBP) was the most active symbol on the TSX.V...